10+ Does Japan Require Iec-60601 4Th Edition
The motto of the worldwide Scout Movement Be Prepared is a good mantra for all aspects of life and in business is undeniably the key to success. The work on IEC 60601-1 Edition 4 is also in the works and is currently expected to be published around 2025 but will likely be later.

Pdf Medical Electrical Equipment Part 1 General Requirements For Basic Safety And Essential Performance Richard Um Academia Edu
New medical EMC standard IEC 60601-1-2 4th edition The 60601-1 collateral standard for medical EMC is 60601-1-2 presently the 3rd edition of the standard is in force.

. 12 As with any new. This International Standard applies to the BASIC SAFETY and ESSENTIAL PERFORMANCE of MEDICAL ELECTRICAL EQUIPMENT. The standard in and of itself is all about ensuring that basic safety and essential performance of a medical device is maintained in the presence of electromagnetic energy.
When is Compliance required for 4th edition 60601-1-2 From the IEC website for SC62A the committee responsible 60601-1-2 Ed 4 is at the ADIS stage approved for. General requirements for basic safety and essential performance gives general requirements of the. As previously noted the fourth edition applies the principles of the risk management approach presented in edition 31 of IEC 60601-1 the current edition to medical device safety issues.
The standard governing electromagnetic compatibility EMC in medical devices IEC 60601-1-2 4th edition has been in effect for several years. Incorporate the requirements of GB 970615 IEC 60601-1-1 and YY 0708 IEC 60601-1-4 which are to be abolished when GB 970612020 takes effect Transforming GB. Recognized Consensus Standards.
4Be prepared for the 4th edition of the IEC 60601-1 medical standard. Emergency Medical Services are automatically classified as the Home Healthcare Environment per clause 81 of IEC 60601-1-2 4th edition. The risk management process now part of the IEC 60601-1-2 4th edition requires assessment of risk resulting from reasonably foreseeable electromagnetic disturbances This is an.
For medical power supplies the IEC 60601-1-2 is the key standard for EMC for medical devices which is often referred to in Europe as EN 60601-1-2 and in Canada as CSA. In September of 2020 the. The general standard IEC 60601-1 Medical electrical equipment Part 1.
5Understanding the Central Differences between the 3rd and 4th. The standard also indicates that increased test. The fourth edition IECEN 60601-1-2 4 th Edition will become a mandatory standard covering safety for medical devices on December 31 2018.
This is NOT the 4th Edition of the IEC 60601-1. The 4 th edition.

Summer 2013

Boxoffice December 23 1968

Compendium Of Innovative Oms By Javier Camacho Issuu

Pdf Multi Temporal Remote Sensing Of Mass Graves In Temperate Environments Emily A Norton And Paul Cheetham Academia Edu

Iec 60601 Standards Series Electrical Medical Devices Manufacturers

Usability Standard Iec 60601 1 6 In The Medical Device Industry
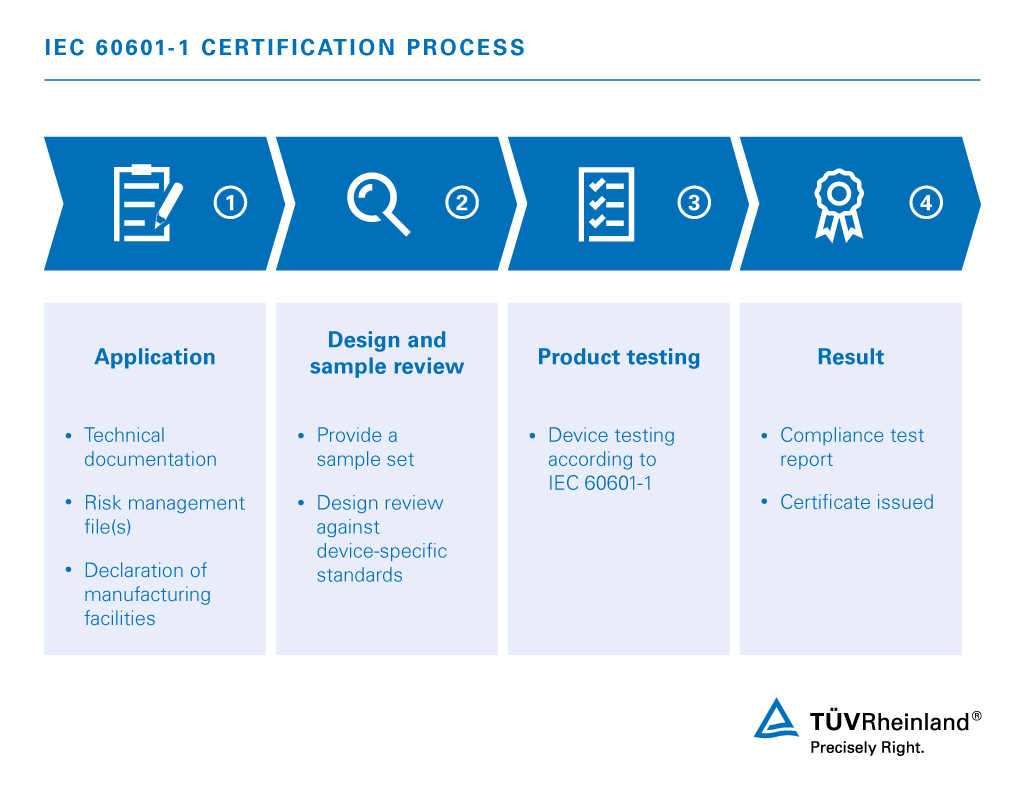
Iec 60601 1 Medical Electrical Equipment Jp Tuv Rheinland

Iec 60601 Product Safety Standards For Medical Devices

The Opportunities Demands And Standards Of Today S Medical Power Supply Market

Iso Iec 9995 Wikiwand
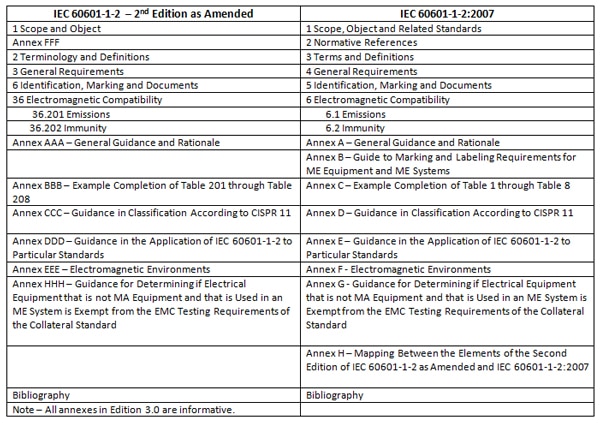
The International Medical Device Emc Standard Iec 60601 1 2 Interference Technology

Dx S Manual Pdf Medical Device Laser

Iec 60601 1 2 4th Edition What You Need To Know Cui Inc
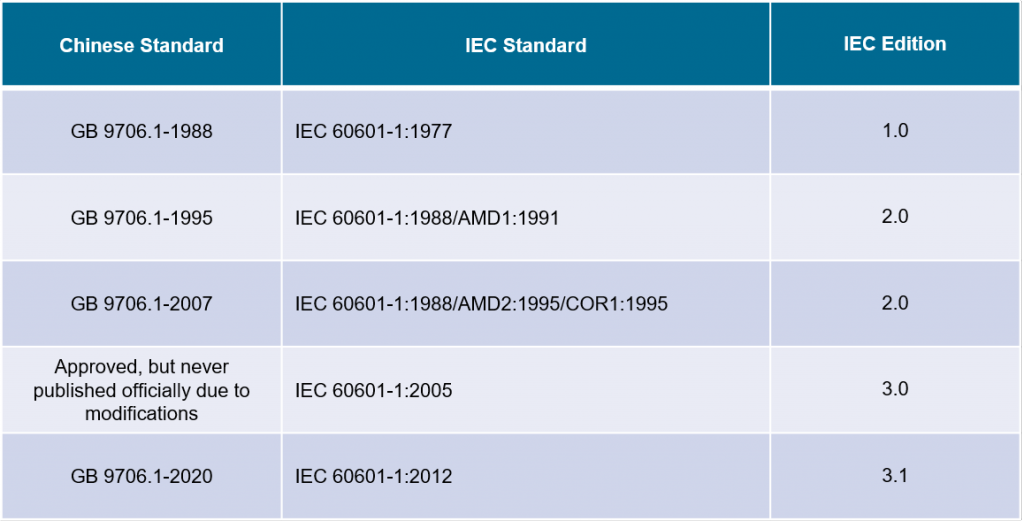
China S New Standard For Medical Electrical Equipment

Pdf Controversies In Medical Physics A Compendium Of Point Counterpoint Debates Ali Moulaei Nejad Academia Edu

National Deviations To Iec 60601 1 Mddionline Com

Mechanical Design Engineer Resume Samples Velvet Jobs